
 Merck’s New mAb Against IL-23 Achieves Clinical Success in Psoriasis
Merck’s New mAb Against IL-23 Achieves Clinical Success in Psoriasis
05Mar13
A report on MedPage Today describes the preliminary result of a randomized, controlled, phase IIb study of the efficacy of an anti-IL-23 monoclonal antibody in the treatment of chronic plaque psoriasis presented at the annual meeting of the American Academy of Dermatology in Miami. The antibody is a humanized IgG1 monoclonal directed towards the p19 subunit of IL-23 and not the p40 subunit shared with IL-12.
Patients (comprised of adults diagnosed with plaque psoriasis of duration ≥6 months, a PASI score ≥12, body surface area (BSA) involvement ≥10%, and a PGA of at least 3) were randomized to placebo or to one of four doses of MK-3222 (5, 25, 100, or 200 mg) and placebo. Patients received their group-specific injections at baseline, week 4, week 12, and then every 12 weeks until week 52 (similar to the dosing regimen for the approved anti-IL-12/IL-23 mAb, Stelara). The primary endpoint was PASI-75 response rate after 16 weeks. PASI-75 response rates were 4.4% with placebo, 33.3% with 5 mg of MK-3222, 64.4% with 25 mg, 66.3% with 100 mg, and 74.4% with 200 mg, the latter three doses giving higher response rates than placebo that were statistically significant. No dose-dependent toxicity was observed and the drug was deemed safe and well tolerated.
There are two main types of biologics (see this link) approved for the treatment of psoriasis. Enbrel (etanercept), Humira (adalimumab), Remicade (infliximab) and Simponi (golimumab) are drugs that block TNF-alpha while Stelara (ustekinumab) targets IL-12 and 1L-23 through their shared p40 subunit. As noted in a recent article, at twelve weeks the “Weighted average PASI-75 scores for infliximab, ustekinumab, adalimumab, etanercept, and alefacept were 78.6%, 72.1%, 70.5%, 48.1%, and 21%, respectively".
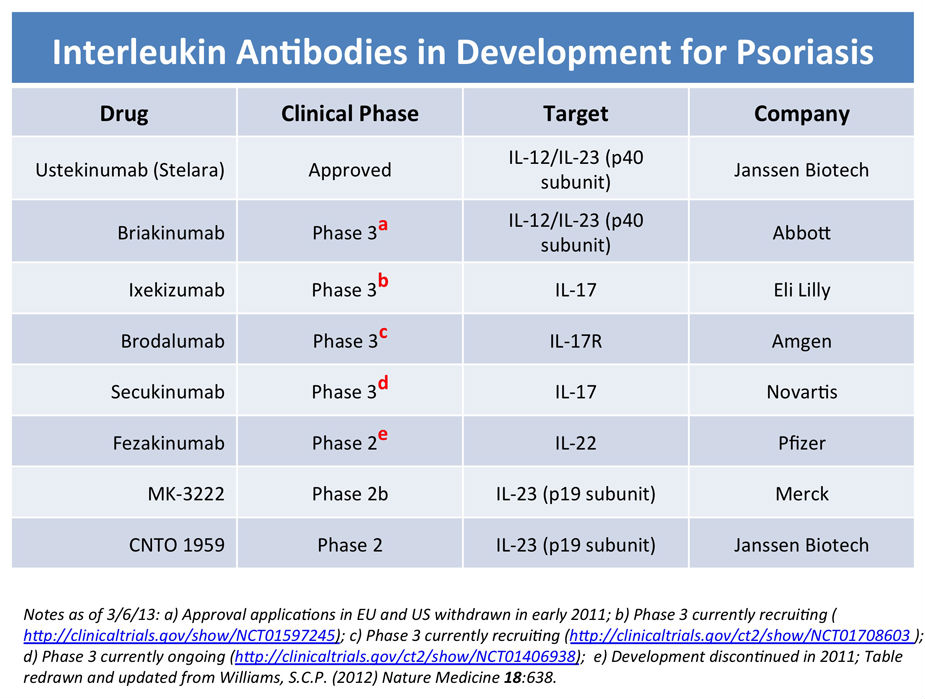
This places the Merck antibody (with it’s 16 week score] within the range of the best of the current biological for psoriasis but potentially with lower toxicities than the currently approved battalion of biologics. As noted last year in a news article in Nature Medicine, the therapeutic landscape continues to evolve for psoriasis. The original set of TNF blockers suffer from the fact that they lose potency over time and can cause GI or liver problems. The next generation of biologics is seeking to target IL-17 and IL-17R since Th17 helper T cells are present in psoriatic lesions at high levels. The first of these Th17 class of biologics for psoriasis to be approved was Stelara, which targets IL-23 activity that promotes Th17 cell production. MK-3222 is one of a host candidate biologics currently in trials that is seeking to selectively block interleukins involved in the Th17 pathway more effectively and with fewer side effects and lower toxicity (see summary, below, redrawn from the Nature Medicine article).
 Xeljanz Notches a Post-Approval Win; Reduces Progression of RA Structural Joint Damage
Xeljanz Notches a Post-Approval Win; Reduces Progression of RA Structural Joint Damage
28Feb13
The JAK kinase inhibitor, tofacitinib (a.k.a. Xeljanz) was initially approved last fall to treat adults with moderately- to severely-active rheumatoid arthritis (RA) who have had an inadequate response to, or who are intolerant of, methotrexate (MTX). At the time, the safety and efficacy of Xeljanz had been evaluated in seven clinical trials in adult patients with moderately to severely active RA. In all of the trials, patients treated orally with Xeljanz experienced improvement in clinical response and physical functioning compared to patients treated with placebo.
However, Xeljanz was associated with an increased risk of serious infections, including opportunistic infections and TB, as well as cancers and lymphoma. Xeljanz treatment is also associated with increases in cholesterol and liver enzyme test levels and decreases in blood counts. The approved labeling recommended a dosage of 5 mg taken twice daily.
Lingering safety concerns prompted the FDA to require a post-marketing study to study the long-term effects of Xeljanz on heart disease, cancer, and serious infections. One such study was already under way: an international, 24-month, double-blind, parallel-group, placebo-controlled phase III study to examine structural preservation in patients with active RA receiving background MTX. Two doses of Xeljanz were being evaluated: the label-recommended 5 mg (b.i.d) and a higher 10 mg (b.i.d.) dose. The study is employing 797 patients with active disease.
As reported by Nancy Walsh in MedPage Today, results from a 12 month interim analysis have now been published in the March edition of Arthritis & Rheumatism. In brief, the analysis found that “…. tofacitinib inhibits progression of structural damage and improves disease activity in patients with RA who are receiving MTX.” However, statistically significant benefits were seen at in radiological scoring at 6 months only for the higher dose group, though this may be a design limitation of the study. In addition, “After a year of treatment, the ACR20, 50, and 70 responses in the 5-mg group were 48.5%, 32.7%, and 18.8%, respectively, and 57%, 41.1%, and 27.5% for the 10-mg group.” The safety profile was consistent with findings in previous studies.
Blocking the progression of joint damage in RA would be a big deal, as joint damage is permanent and causes joints to not work well even outside of flares. While most DMARDs and biological DMARDs slow joint damage progression, the current TNF blockers do the best job, essentially “uncoupling” inflammation from joint destruction. Indeed, joint inflammation minus joint destruction and joint preservation minus control of inflammation may constitute two endpoints in the spectrum of RA. The interim analysis provided here provides preliminary evidence for potential additional benefit for RA patients taking tofacitinib, but so far only at the higher (10 mg b.i.d.) dose. Given the existing safety concerns at the recommended 5 mg b.i.d. dose, it will be interesting to monitor how RA patients and physicians balance risk and benefit with the orally-available Xeljanz, assuming confirmation of these interim results, versus the use of biologics.
 Vitamin D Strikes Out in 1st Clinical Trial for Respiratory Infections
Vitamin D Strikes Out in 1st Clinical Trial for Respiratory Infections
02Oct12
An article appearing in Medscape Infectious Disease describes the results of a randomized, placebo-controlled trial which shows that "...adding vitamin D supplements to your diet with will not prevent upper respiratory tract infections (URTIs) or hasten your recovery from them". The results of the clinical trial will be published online October 3 in the Journal of the American Medical Association.
This is the first definitive clinical trial to determine whether vitamin D therapy reduces rates of upper respiratory tract infections in adults. The results stand in contrast to a variety of observational studies, as well as basic research, that indicated a possible benefit from Vitamin D against respiratory infections in humans.
 Meanwhile, A Mixed Bag for Vitamin D
Meanwhile, A Mixed Bag for Vitamin D
05Sep12
A couple of recent reports yield a mixed bag for therapeutic uses of Vitamin D. On the one hand, supplementing vitamin D-deficient individuals (where Vit. D deficiency is associated with dyslipidemia) did NOT improve lipid profiles. This is the observation from a randomized, placebo-controlled trial where patients received an oral dose of 50,000 IU of Vitamin D weekly for eight weeks. In fact, vitamin supplementation led to worsened serum indicators. The full study is published in Arteriosclerosis, Thrombosis, and Vascular Biology and can be found here.
On the other hand, the BBC is reporting that vitamin D may be useful in treating tuberculosis, citing a recent paper just out in The Proceedings of the National Academy of Sciences. Specifically, the new study found that patients given vitamin D in combination with antibiotics recovered more quickly from TB than those given antibiotics alone.
As noted by The Times of India, vitamin D was used years ago for the treatment of TB, a treatment regimen known as heliotherapy or “forced sunbathing”. Use of Vita. D for TB was dropped upon the advent of antibiotics.
How does Vitamin D work against lung infections, like TB? Vitamin D apparently alters the host’s inflammatory response during the infection. While inflammation is a normal and an important part of the body's response to infection, its actions create a new opportunity for lung pathogens. For example, during TB infection, inflammation remodels the scaffolding in the lungs to allow infection-fighting white blood cells in. But this remodeling also creates tiny cavities in the lungs in which TB bacteria can hide. Vitamin D helps accelerate resolution of the inflammatory response, thereby reducing the extent of scaffold remodeling.
 Statin Retards Progressive MS
Statin Retards Progressive MS
10Oct12
MedPage Today, covering the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis, is reporting on a clinical trial which provides preliminary evidence that high dose Zocor (simvastatin) “significantly reduced brain atrophy and slowed advancement of disability for 2 years in patients with secondary progressive multiple sclerosis..” The study was presented by Dr. Jeremy Chataway (University College London) and was comprised of a 140 patient, randomized format with certain patients receiving 80 mg/day of Zocor for 2 years.
“Significant reductions in disability progression, as measured by EDSS and MS Impact Score (MSIS), were also seen with simvastatin in the trial, he told attendees at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis. On the other hand, differences between the treatment groups in specific functional outcomes, such as walking ability and hand dexterity, did not reach statistical significance. “ Others cited in the report also point out that brain atrophy is a surrogate marker for progressive MS and that more clinically relevant endpoints are needed before widespread use is approved.
 The Anti-inflammatory Vitamin B3 Protects Mice from Staph a.
The Anti-inflammatory Vitamin B3 Protects Mice from Staph a.
28Aug12
A report in Genetic Engineering & Biotechnology News describes new evidence that vitamin B3, already prescribed as a topical agent for certain inflammatory disorders, enhances the ability of the innate immune system to confront Staphylococcus aureus infections in a therapeutically meaningful manner. A well-known immuno-modulatory agent, nicotinamide has previously been shown to be effective as an anti-TB agent and effective (enhanced survival) in experimental murine models of Gram-positive and Gram-negative sepsis.
Citing a paper that appeared last month in the Journal of Clinical Investigation, the GEN article describes how administration of vitamon B3 (nicotinamide) alone, either prophylatically or therapeutically, enhanced the clearance of S. aureus by up to 1000-fold. Mice systemically-infected with S. aureus and treated with nicotinamide starting 12 hours post-infection were cleared of infection 60 hours later and the number of residual bacteria recovered from spleen or kidneys was reduced 30-1000 fold versus untreated controls.
Nicotinamide apparently exerts its effects by ehancing the expression of the myeloid transcription factor C/EBPε [ CCAAT/enhancer-binding protein ε], especially in neutrophils. Prior work with C/EBPε-deficient mice showed that isolated neutrophils display aberrant phagocytosis, respiratory burst, and bactericidal activities, essentially a phenotype similar to that of individuals with Neutrophil-Specific Granule Deficiency. Enhanced expression of C/EBPε is believed to occur as a result of competitive inhibition of class III histone deacetylase(s) [HDACs] by nicotinamide. Prior genome-wide expression analysis has already indicated an important role for HDACs in host defense.
The authors of the JCI paper argue that this paper demonstrates "....that compounds exerting modulatory effects of the myeloid-specific transcription factor C/EBPε may be suitable candidates for antimicrobial therapeutics".
© 2012-2014 Acton Biotech Consulting - See Image Credits page for attribution and license conditions for non-original images/media.
Important DISCLAIMER - This is a science & technology website and not a medical treatment or diagnostic site. No information contained within this site is a substitute for advice or direction given by qualified medical professionals, nor is it intended to inform patients regarding treatment options or disease diagnosis/prognosis. As always, individuals should consult their own medical team about issues concerning their health and well being.